Ellagic acid and urolithin A
Ellagic acid is a polyphenol that is found in a lot of fruits and vegetables, among others. (For those interested: berries of all types, walnuts, and pomegranate are all very rich in ellagic acid.)
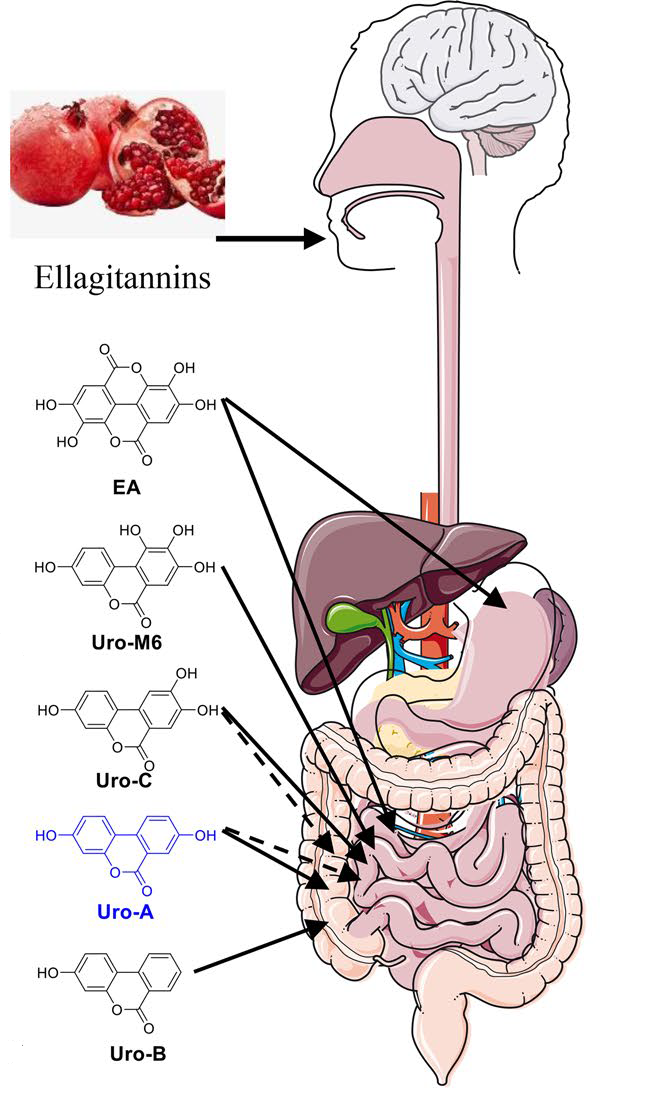
In 1980, scientists observed that EA was converted into a compound called urolithin A in the rat gut microbiome. Only in 2005, the same process was observed in humans.
Cool, why should we care?
Well, urolithin A regulates mitophagy or the recycling of damaged mitochondria. As a result, urolithin A supports overall mitochondrial health and safeguards our cellular energy production. It also seems to have some anti-inflammatory properties, can improve cholesterol metabolism, and possibly downregulate amyloid plaque formation.
These properties of urolithin A have led scientists to suggest that it could be a healthy longevity-promoting molecule. For example, a human trial with urolithin A in elderly participants found a few modest positive effects on inflammation and mitochondrial health. A placebo-controlled intervention trial supported these findings.
So, should we make sure to get a lot of ellagic acid in our diets? Sure, but there is still a significant catch, though.
There is quite a bit of individual variability in how efficiently people's bodies can convert ellagic acid into urolithin A (which partially depends on gut microbiome composition). That conversion efficiency goes down as we age. Roughly 40% of the elderly population are good 'UA producers', which means 60% are not.
Transformation
A 2022 review takes the magnifying glass to how ellagic acid becomes urolithin A and what the microbiome has to do with it.
There are a lot of steps involved in going from ellagic acid to urolithin A, and many enzymes are involved. In other words, there remain uncertainties about the process. But here is what we do know:
- Ellagic acid first undergoes a process that removes a chunk of its chemical structure (a lactone ring), forming Uro-M5 in the colon.
- Then, hydroxyl groups ( — OH) are chopped off at different positions. This produces something called tetrahydroxy-urolithins. Getting closer.
- Another hydroxyl group is removed, leading to the formation of trihydroxy-urolithins. another step closer to urolithin A.
- Moving on through our intestines, a dihydroxylation reaction follows, resulting in dihydroxy-urolithins, including — you guessed it — urolithin A.
- (This urolithin A can go on to lose another hydroxyl group to become a monohydroxy-urolithin known as urolithin B. This even less researched urolithin also seems to have some health benefits, but we lack good data on this, especially in humans.)
Many uncertainties remain. Are there alternative pathways? How hard is it to produce urolithin B from urolithin A and what's the limiting factor in this conversion? Which enzymes are involved in each step?
So far, it seems that an enzyme with the tongue-twisting name pyrocatechol-dehydroxylase is the primary enzyme involved, and its diversity in intestinal microflora accounts for variations in the dehydroxylation steps. Or, some microbes produce more of this enzyme, which then might improve ellagic acid to urolithin A conversion.
Which microbes, you ask? The data we have so far implicates little critters belonging to the group of bacteria called Gordinobacter. Preliminary tests in mice suggest that these specific strains of these bacteria might serve as a probiotic to boost urolithin A production (although interactions with the existing microbiome might mitigate these effects).
I think I'm going to snack on berries, pomegranate, and some nuts now.

